See also tabulated values of specific heat of gases food and foodstuff metals and semimetals common liquids and fluids and other common substances as well as values of molar heat capacity of common organic substances and inorganic substances.
Specific heat of glass marbles lab.
The specific heat of water is 4 200 j kg c.
As an example the specific heat of water is given as which means that 1 00 calorie of heat is necessary to raise one gram of water one degree celsius or 4190 joules of heat are necessary to raise one kilogram of water one kelvin.
The specific heat of a glass is the heat needed to raise the temperature of the glass by 1 c per unit weight.
Specific heat is the amount of energy measured in joules needed to raise the temperature of one gram of the substance one celsius degree.
Often applied to metallic elements specific heat can be used as a basis for comparing how different substances absorb and transfer energy.
Chapter 12 lecture notes pdf textbook chapter 12 pdf class worksheets.
A final activity will assess students understanding of specific heat and heat capacity and promote data analysis skills using real life situations.
And lead with thread stand and clamp x2 glass rod lab jack x2.
Where q is heat m is mass and t is temperature.
A separate video will show you the calculation that wi.
If the thermal conductivity shows how much heat will flow through a material the specific heat shows how quickly heat will raise the temperature of a glass.
Specific heat values for various other materials are listed in table 1 below.
The formula can then be rearranged for determining the specific heat of the sample.
This activity is a chemistry lab based investigation where students apply observational skills and critical thinking skills to finding specific heat and heat capacity using different temperatures of water and solids.
Background the specific heat of a substance usually indicated by the symbol c is the amount of heat required to raise the temperature of one kilogram of the substance by one degree centigrade.
Q w q s where q is the change in heat.
Q w c w m w t peak t initial w q s c s m s t peak t initial s.
Part of ncssm core collection.
This video takes you through the major steps in the specific heat of metals lab that we will be doing.
The specific heat of some commonly used solids is given in the table below.
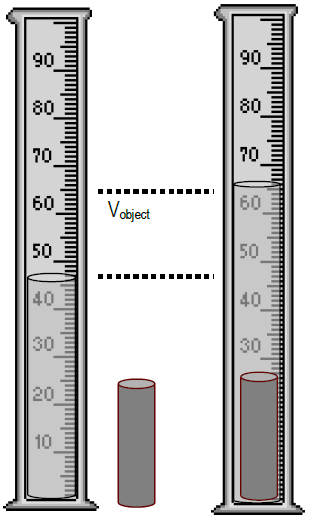
To measure specific heat in the laboratory a calorimeter of some.
Intermolecular forces video.
Http www dlt ncssm edu please attribute t.
For conversion of units use the specific heat online unit converter.
C s c w m w δt w m s δt.
Using specific heat of water and the mass of water being used we can express the above more informatively.